Open Hardware Electrophoresis Equation,Wood Carving Machine Bunnings Video,Woodwork Projects Simple Level,Concealed Wardrobe Door Hinges - And More

This latter force is not actually applied to the particle, but to the ions in the diffuse layer located at some distance from the particle surface, and part of it is transferred all the way to the particle surface through viscous stress.
This part of the force is also called electrophoretic retardation force. When the electric field is applied and the charged particle to be analyzed is at steady movement through the diffuse layer, the total resulting force is zero :.
The most well known and widely used theory of electrophoresis was developed in by Smoluchowski : [11]. The Smoluchowski theory is very powerful because it works for dispersed particles of any shape at any concentration. It has limitations on Open Hardware Electrophoresis Wikipedia its validity.
However, Debye length must be important for electrophoresis, as follows immediately from the Figure on the right. Increasing thickness of the double layer DL leads to removing the point of retardation force further from the particle surface. The thicker the DL, the smaller the retardation force must be.
Detailed theoretical analysis proved that the Smoluchowski theory is valid only for sufficiently thin DL, when particle radius a is much greater than the Debye length:. This model of "thin double layer" offers tremendous simplifications not only for electrophoresis theory but for many other electrokinetic theories.
This model is valid for most aqueous systems, where the Debye length is usually only a few nanometers. It only breaks for nano-colloids in solution with ionic strength close to water.
The Smoluchowski theory also neglects the contributions from surface conductivity. This is expressed in modern theory as condition of small Dukhin number :. In the effort of expanding the range of validity of electrophoretic theories, the opposite asymptotic case was considered, when Debye length is larger than particle radius:.
This model can be useful for some nanoparticles and non-polar fluids, where Debye length is much larger than in the usual cases. There are several analytical theories that incorporate surface conductivity and eliminate the restriction of a small Dukhin number, pioneered by Overbeek.
In the thin double layer limit, these theories confirm the numerical solution to the problem provided by O'Brien and White. From Wikipedia, the free encyclopedia. Motion of charged particles in electric field. For specific types and uses of electrophoresis for example, in various analytical methods and as a process of administering medicine, iontophoresis , see Electrophoresis disambiguation. Main article: History of electrophoresis.
Affinity electrophoresis Electrophoretic deposition Capillary electrophoresis Dielectrophoresis Electroblotting Gel electrophoresis Gel electrophoresis of nucleic acids Immunoelectrophoresis Isoelectric focusing Isotachophoresis Pulsed-field gel electrophoresis Nonlinear frictiophoresis. Fundamentals of Interface and Colloid Science. Foundations of Colloid Science. Oxford University Press. Electrokinetic Phenomena. Wiley and Sons. Colloidal Dispersions.
Cambridge University Press. Colloid Science. Volume 1, Irreversible systems. Because the interior of the capillary is cylindrical, the length of the sample, l , is easy to calculate using the equation for the volume of a cylinder; thus.
Suppose you need to limit your injection to less than 0. The capillary is 75 cm long, which means that 0. To convert this to the maximum volume of sample we use the equation for the volume of a cylinder. In an electrokinetic injection we place both the capillary and the anode into the sample and briefly apply an potential.
The electric field in the sample is different that the electric field in the rest of the capillary because the sample and the buffer have different ionic compositions.
The effective electric field is. This method of injection is called stacking. Stacking is accomplished by placing the sample in a solution whose ionic strength is significantly less than that of the buffer in the capillary tube. As a result, the cations in the sample plug migrate toward the cathode with a greater velocity, and the anions migrate more slowly—neutral species are unaffected and move with the electroosmotic flow.
When the ions reach their respective boundaries between the sample plug and the buffer, the electrical field decreases and the electrophoretic velocity of the cations decreases and that for the anions increases. Over time, the buffer within the capillary becomes more homogeneous and the separation proceeds without additional stacking. Migration in electrophoresis occurs in response to an applied electric field.
Because narrow bored capillary tubes dissipate Joule heating so efficiently, voltages of up to 40 kV are possible. Most of the detectors used in HPLC also find use in capillary electrophoresis. Detection limits are about 10 —7 M. Better detection limits are obtained using fluorescence, particularly when using a laser as an excitation source. Emission is measured at an angle of 90 o to the laser. Because the laser provides an intense source of radiation that can be focused to a narrow spot, detection limits are as low as 10 —16 M.
Characteristics of Detectors for Capillary Electrophoresis. There are several different forms of capillary electrophoresis, each of which has its particular advantages.
Four of these methods are described briefly in this section. The simplest form of capillary electrophoresis is capillary zone electrophoresis. In CZE we fill the capillary tube with a buffer and, after loading the sample, place the ends of the capillary tube in reservoirs that contain additional buffer.
Usually the end of the capillary containing the sample is the anode and solutes migrate toward the cathode at a velocity determined by their respective electrophoretic mobilities and the electroosmotic flow.
Cations elute first, with smaller, more highly charged cations eluting before larger cations with smaller charges. Neutral species elute as a single band.
Anions are the last species to elute, with smaller, more negatively charged anions being the last to elute. We can reverse the direction of electroosmotic flow by adding an alkylammonium salt to the buffer solution. The tail of the alkyl ammonium ion is hydrophobic and associates with the tail of another alkyl ammonium ion. The result is a layer of positive charges that attract anions in the buffer.
The order of elution is exactly opposite that observed under normal conditions. In this form of CZE the cations migrate from the anode to the cathode.
Anions elute into the source reservoir and neutral species remain stationary. Capillary zone electrophoresis provides effective separations of charged species, including inorganic anions and cations, organic acids and amines, and large biomolecules such as proteins.
For example, CZE was used to separate a mixture of 36 inorganic and organic ions in less than three minutes [Jones, W. A mixture of neutral species, of course, can not be resolved. One limitation to CZE is its inability to separate neutral species.
Sodium dodecylsulfate, or SDS, consists of a long-chain hydrophobic tail and a negatively charged ionic functional group at its head. When the concentration of SDS is sufficiently large, above the critical micelle concnetration, a micelle forms.
Because SDS micelles have a negative charge, they migrate toward the cathode with a velocity less than the electroosmotic flow velocity. Neutral species partition themselves between the micelles and the buffer solution in a manner similar to the partitioning of solutes between the two liquid phases in HPLC. Because there is a partitioning between two phases, we include the descriptive term chromatography in the techniques name.
Note that in MEKC both phases are mobile and positively charged micelles can be formed using cetylammonium bromide. While the micelles have a negative charge, they are carried from the anode to the cathode due to the eof , albeit at a slower rate because their migration towards the anode.
Image source currently unknown. The elution order for neutral species in MEKC depends on the extent to which each species partitions into the micelles.
Neutral solutes that are extremely hydrophobic are completely soluble in the micelle, eluting with the micelles as a single band. Those neutral species that exist in a partition equilibrium between the buffer and the micelles elute between the completely hydrophilic and completely hydrophobic neutral species.
Those neutral species that favor the buffer elute before those favoring the micelles. Micellar electrokinetic chromatography is used to separate a wide variety of samples, including mixtures of pharmaceutical compounds, vitamins, and explosives.
The addition of micelles formed from chiral additives combined with the tremendous separation efficiency of capillary electrophoresis due to the plug flow yields the most powerful methods for chiral separations. Image source Analytical Chemistry, Vol.
In capillary gel electrophoresis the capillary tubing is filled with a polymeric gel. Because the gel is porous, a solute migrates through the gel with a velocity determined both by its electrophoretic mobility and by its size. The ability to effect a separation using size is helpful when the solutes have similar electrophoretic mobilities.
For example, fragments of DNA of varying length have similar charge-to-size ratios, making their separation by CZE difficult. The capillary used for CGE usually is treated to eliminate electroosmotic flow to prevent the gel from extruding from the capillary tubing.
Samples are injected electrokinetically because the gel provides too much resistance for hydrodynamic sampling. The primary application of CGE is the separation of large biomolecules, including DNA fragments, proteins, and oligonucleotides. Another approach to separating neutral species is capillary electrochromatography. In CEC the capillary tubing is packed with 1. The best way to appreciate the theoretical and the practical details discussed in this section is to carefully examine a typical analytical method.
Although each method is unique, the following description of the determination of a vitamin B complex by capillary zone electrophoresis or by micellar electrokinetic capillary chromatography provides an instructive example of a typical procedure. The description here is based on Smyth, W. The water soluble vitamins B 1 thiamine hydrochloride , B 2 riboflavin , B 3 niacinamide , and B 6 pyridoxine hydrochloride are determined by CZE using a pH 9 sodium tetraborate-sodium dihydrogen phosphate buffer, or by MEKC using the same buffer with the addition of sodium dodecyl sulfate.
Detection is by UV absorption at nm. An internal standard of o -ethoxybenzamide is used to standardize the method. Crush a vitamin B complex tablet and place it in a beaker with After mixing for 2 min to ensure that the B vitamins are dissolved, pass a 5. For CZE the capillary column contains a 20 mM pH 9 sodium tetraborate-sodium dihydrogen phosphate buffer.
Methanol, which elutes at 4. When using standard solutions of each vitamin, CZE peaks are found at 3. At a pH of 9, vitamin B 1 is a cation and elutes before the neutral species methanol; thus it is the compound that elutes at 3. Vitamin B 3 is a neutral species at a pH of 9 and elutes with methanol at 4. The remaining two B vitamins are weak acids that partially ionize to weak base anions at a pH of 9.
Of the two, vitamin B 6 is the stronger acid a p K a of 9. Vitamin B 6 , therefore, is the last of the vitamins to elute. What conclusions can you make about the solubility of the B vitamins in the sodium dodecylsulfate micelles? The micelles elute at The elution time for vitamin B 1 shows the greatest change, increasing from 3. Clearly vitamin B 1 has the greatest solubility in the micelles.
Vitamin B 2 and vitamin B3 have a more limited solubility in the micelles, and show only slightly longer elution times in the presence of the micelles. Interestingly, the elution time for vitamin B 6 decreases in the presence of the micelles. For quantitative work an internal standard of o -ethoxybenzamide is added to all samples and standards.
Why is an internal standard necessary? Although the method of injection is not specified, neither a hydrodynamic injection nor an electrokinetic injection is particularly reproducible. The use of an internal standard compensates for this limitation. When compared to GC and HPLC, capillary electrophoresis provides similar levels of accuracy, precision, and sensitivity, and it provides a comparable degree of selectivity.
The amount of material injected into a capillary electrophoretic column is significantly smaller than that for GC and HPLC—typically 1 nL versus 0. The most significant advantages of capillary electrophoresis are improvements in separation efficiency, time, and cost. Separations in capillary electrophoresis are fast and efficient. Theory of Electrophoresis In capillary electrophoresis we inject the sample into a buffered solution retained within a capillary tube.
Schematic diagram showing the origin of the double layer within a capillary tube. Although the net charge within the capillary is zero, the distribution of charge is not. The walls of the capillary have an excess of negative charge, which decreases across the fixed layer and the diffuse layer, reaching a value of zero in bulk solution.
Visual explanation for the general elution order in capillary electrophoresis.


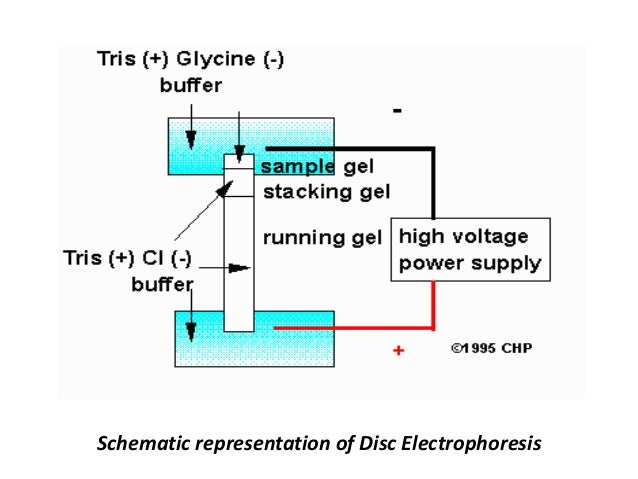
|
Rikon Lathe Duplicator Merillat Cabinet Drawer Slide Parts Simple Woodturning Tools Coupon Receipt |
123321
28.03.2021 at 22:27:48
sex_qirl
28.03.2021 at 10:36:43